Do you remember when we discussed the concept of scientific “ontology” and its significance in data processing and organization? We compared it to organizing a kitchen, where ingredients are meticulously arranged based on their fundamental properties and relationships. In that post on our InnovaBlog, we explained how ontologies help us understand the intricate nature of information, turning raw data into meaningful insights.
In our previous work, we created PaCCO, an ontology that records the effects of 530 drugs during preclinical trials, and this resulted in a publication that you may consult freely here. In brief, we used two ontologies, the Human Phenotype Ontology (HP) and the Mammalian Phenotype Ontology (MP), which have different structures and labels and had missing terms. For this reason, we needed to merge these two ontologies into a unique structure. Nevertheless, obtaining a unified ontological structure with a biological or toxicological meaning is far from trivial, although it is ideal for performing data analysis (e.g., concordance and correlations) to explore the significance of animal and human effects of drugs and chemicals.
Tina Basiripanah, our bioinformatics intern, took on this challenge with a fresh perspective. She worked for several months programming Python codes to carry out our idea of building an entirely new ontology from the ground up, using a structured and systematic approach. The structure of our new ontology has been projected and discussed through our team, obtaining the following multilevel organization:
- Level 1: Systems and apparatus (including a “no system” category when the effects are not linked to a system) in which the effect takes place;
- Level 2: Specific organs affected within each system (including a “no organ” category when the effects are not linked to an organ);
- Level 3: Effect basis, categorizing effects by their class, which reflects the field of analysis used for their detection (e.g., clinical biochemistry, hematology, histopathology, etc.);
- Level 4: Effect, the description of the effect in an ontologized format reflecting the outcome of an experiment.
Beyond the fourth level, in the future, we are also planning to add additional layers, for example, to qualify and quantify effects (e.g., increase, decrease).
Tina’s work resulted in a well-structured and comprehensive ontology that perfectly integrates the terms derived from MP and HP while preserving the specificity of each effect. Take a look at a graphical result of her work!
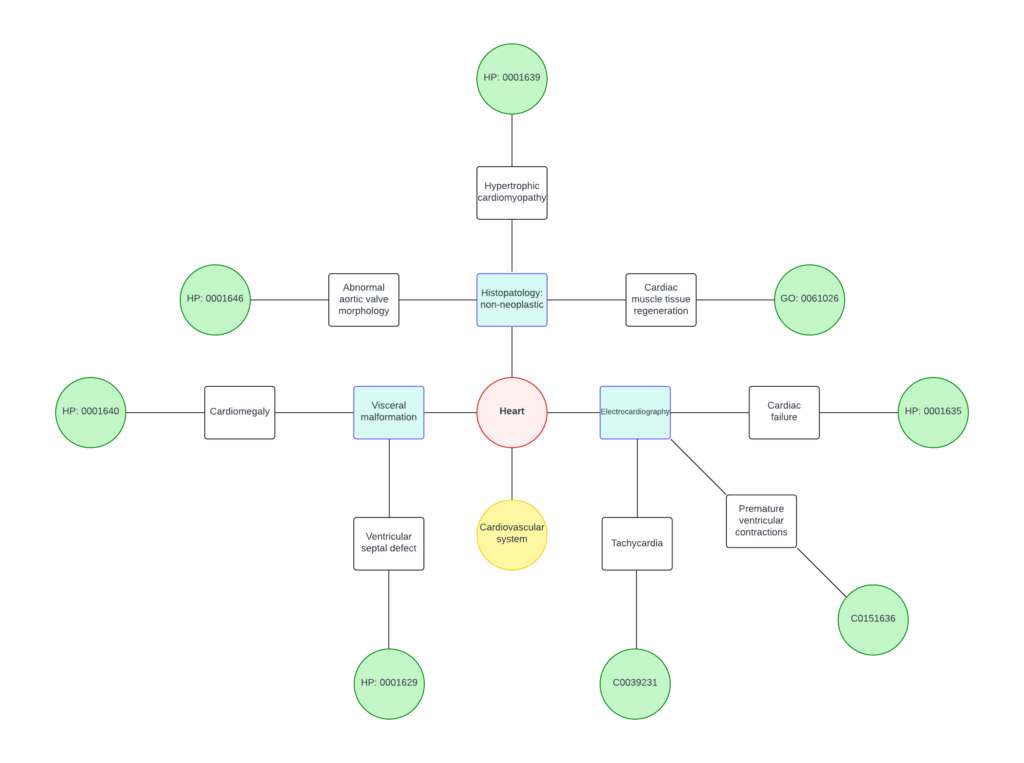
This new ontology not only addresses the gaps between the previous systems but also lays a solid foundation for future expansions and modifications. The creation of this new ontology has significant implications for enhanced data analysis, for example, of toxicological data. Get in touch with us if you are interested in this work!
“Science is organized knowledge; wisdom is organized life” – Immanuel Kant