In a recent study that was published in Frontiers in Toxicology, part of our team alongside ECHA’s experts exploited the results of a previous study on the effects of a list of drugs, focusing on carcinogenicity data from non-clinical animal studies. These data were already included in the database, which is accessible via the IUCLID6 website, but they have been now analysed from another perspective.
A key component of evaluating the safety of pharmaceuticals is carcinogenicity testing, which usually involves long-term rodent studies to determine a drug’s likelihood to cause cancer. These studies can last up to two years in rodents as this duration allows researchers to monitor the development of tumours over a significant portion of the animals’ lifespan, which is crucial for assessing the potential carcinogenic risk to humans. Indeed, the objective of the studies is to find any possible effects that could lead to cancer. However, they often reveal a broader range of toxicological effects, some of which may not directly result in tumour formation but are nonetheless crucial for understanding a drug’s overall safety profile.
The database presented in our latest publication goes beyond merely cataloguing these effects by offering a detailed statistical breakdown. This level of detail allows researchers to examine how various factors may affect a drug’s potential to cause cancer by exploring deeper into the data. The database includes data from 520 studies that evaluate the tumorigenic potential of 238 pharmaceutical compounds. These drugs span various therapeutic areas, including the nervous system, alimentary tract, tumour growth, immune system, and cardiovascular system. It meticulously catalogues tumour occurrences across different species, strains, sexes, and affected organs.
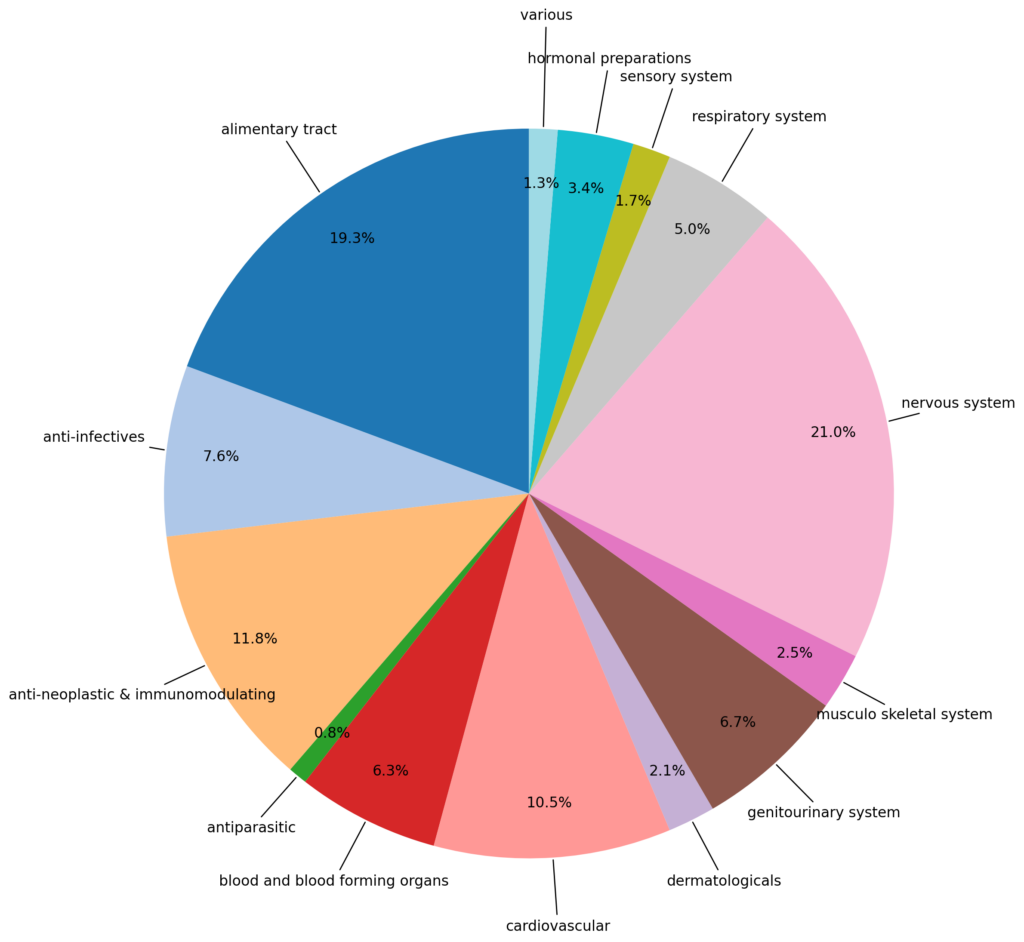
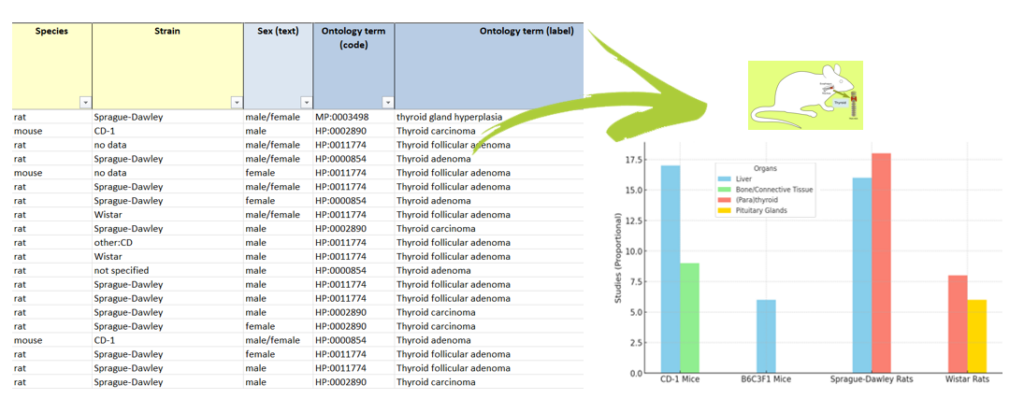
It also contains histopathological changes, which are microscopic changes in tissue that suggest other types of toxicity even though they are not directly related to cancer. Notably, the data reveals specific organ-related trends in tumour occurrence: in CD-1 mice, tumours predominantly affected the liver and bone/connective tissues; in B6C3F1 mice and Sprague-Dawley rats, the liver and (para)thyroid were most commonly impacted; and in Wistar rats, tumours were frequently observed in the (para)thyroid and pituitary glands. These findings underscore the importance of species and organ specificity when assessing the carcinogenic risks of pharmaceuticals or chemicals.
At Innovamol, we are proud to contribute to the global effort to enhance drug safety through scientific data intelligence. This database represented our commitment to such high-quality and actionable data that can drive innovation and ensure the development of safer pharmaceuticals and chemical. We invite researchers, professionals and companies to explore this database and consider its potential to support their work in toxicology. For more details and to access the database, please refer to our publication in Frontiers in Toxicology below or just contact us.
“La scienza è il capitano, e la pratica sono i soldati” (Science is the captain, and practice are the soldiers) – Leonardo da Vinci